Fuel cells have long been considered a promising energy-conversion solution that can generate electricity without the harmful emissions associated with combustion. However, the widespread adoption of fuel cells has been hindered by the expensive materials and precious metal catalysts traditionally used in their design. Recently, researchers from Chongqing University and Loughborough University have made a significant breakthrough in the development of anion-exchange-membrane fuel cells (AEMFCs) that could address these challenges.
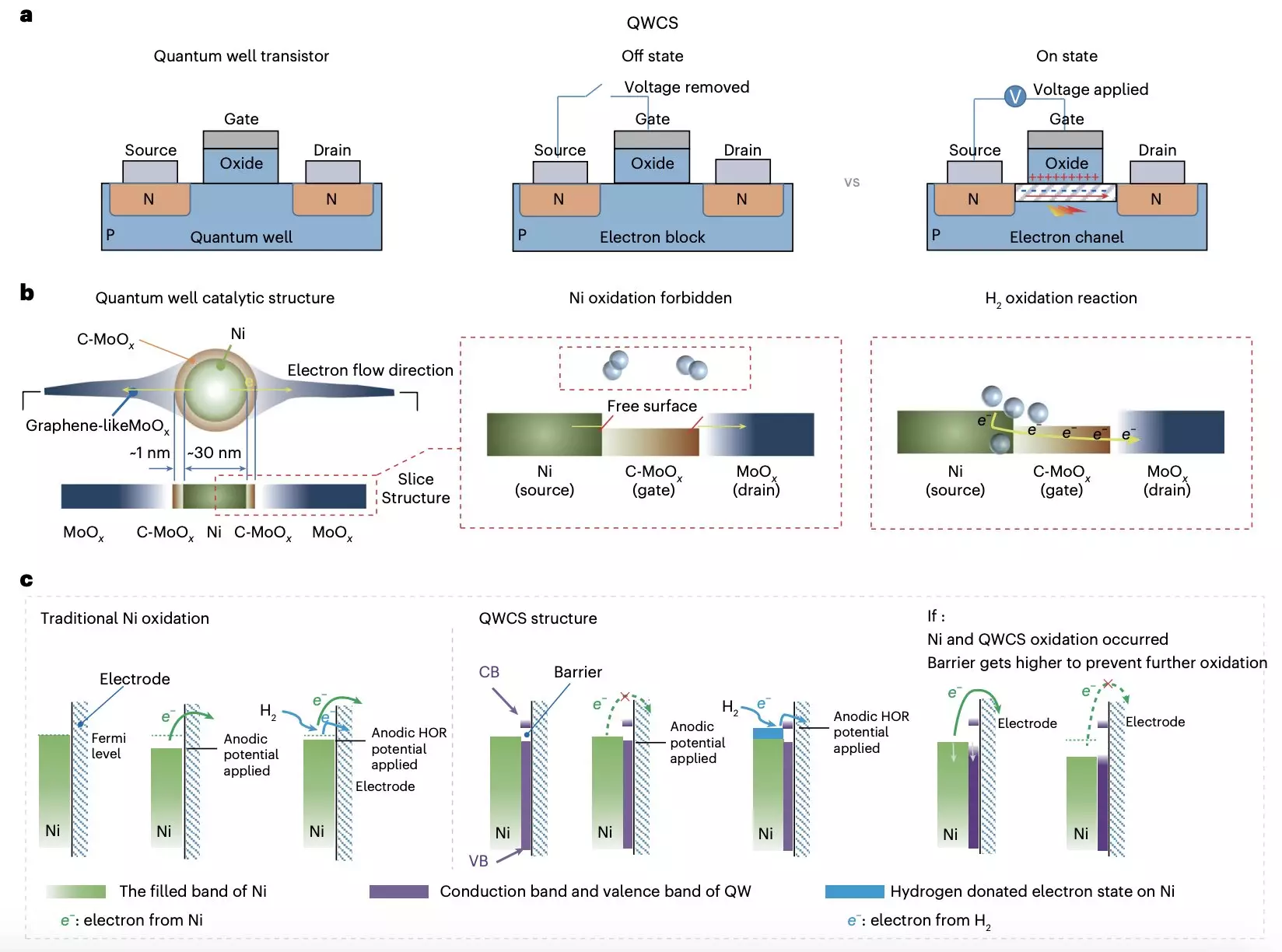
One of the key issues with existing non-precious metal catalysts in AEMFCs is their susceptibility to self-oxidation, which leads to the irreversible failure of the cells. To combat this issue, the research team introduced a novel strategy utilizing a quantum well-like catalytic structure (QWCS) comprised of quantum-confined metallic nickel nanoparticles. This structure, known as Ni@C-MoOx, allows for the selective transfer of external electrons produced during the hydrogen oxidation reaction, without risking the oxidation of the Ni catalyst.
Enhanced Catalytic Activity and Stability
The Ni@C-MoOx catalyst demonstrated excellent stability and catalytic activity, even after 100 hours of continuous operation under harsh conditions. The fuel cell created using this catalyst achieved a high specific power density of 486 mW mgNi-1, with no decline in performance following repeated shutdown-start cycles. The unique design of the QWCS provides a barrier to electrons released from the hydrogen oxidation reaction, ensuring the stability of the Ni nanoparticles and preventing electro-oxidation.
The development of the Ni@C-MoOx catalyst represents a significant step forward in the creation of cost-effective and reliable AEMFCs that do not degrade over time. Furthermore, the underlying design strategy of utilizing quantum confinement to prevent the oxidation of non-precious metals could pave the way for the creation of other innovative catalysts with similar benefits. The research conducted by Zhou, Yuan, and their colleagues opens up new possibilities for the future of fuel cell technology.
The introduction of quantum well-like catalytic structures has the potential to revolutionize the design and performance of fuel cells by addressing the challenges associated with non-precious metal catalysts. The breakthrough achieved by the research team highlights the importance of innovative approaches in developing sustainable energy solutions. As we continue to explore new materials and design strategies, the future of fuel cells looks brighter than ever before.

Leave a Reply