The growing urgency to address climate change has catalyzed innovative research into sustainable energy solutions, particularly in the generation of renewable fuels. Among these avenues is the ambitious field of artificial photosynthesis, where scientists aim to mimic natural processes to convert carbon dioxide (CO2) into usable energy. Recently, a groundbreaking development at the University of Michigan has made headway in this realm, demonstrating a highly effective method for synthesizing ethylene—a vital hydrocarbon that serves as a precursor for various plastics—by utilizing CO2 emissions that would typically contribute to atmospheric pollution.
The pivotal advancement in the Michigan research lies in the ability to link carbon atoms together efficiently. Traditional methods of generating ethylene involve high-pressure and high-temperature processes derived from fossil fuels, which inherently release CO2 into the atmosphere. In contrast, the novel system designed by the University of Michigan team enables the transformation of CO2 directly into ethylene using solar energy. “Our system outshines previously established methods, achieving performance metrics that are five to six times higher than average reported efficiencies in solar-driven CO2 reduction processes,” explains Zetian Mi, the professor leading the project.
This innovation holds promise not just for carbon capture but also for the subsequent transformation of captured CO2 into valuable products, thus actively participating in the creation of a circular economy where waste carbon is utilized rather than emitted. Ethylene’s properties make it an essential building block in the production of plastics, meaning that this technological breakthrough might allow for the sustainable creation of plastic materials from captured greenhouse gases.
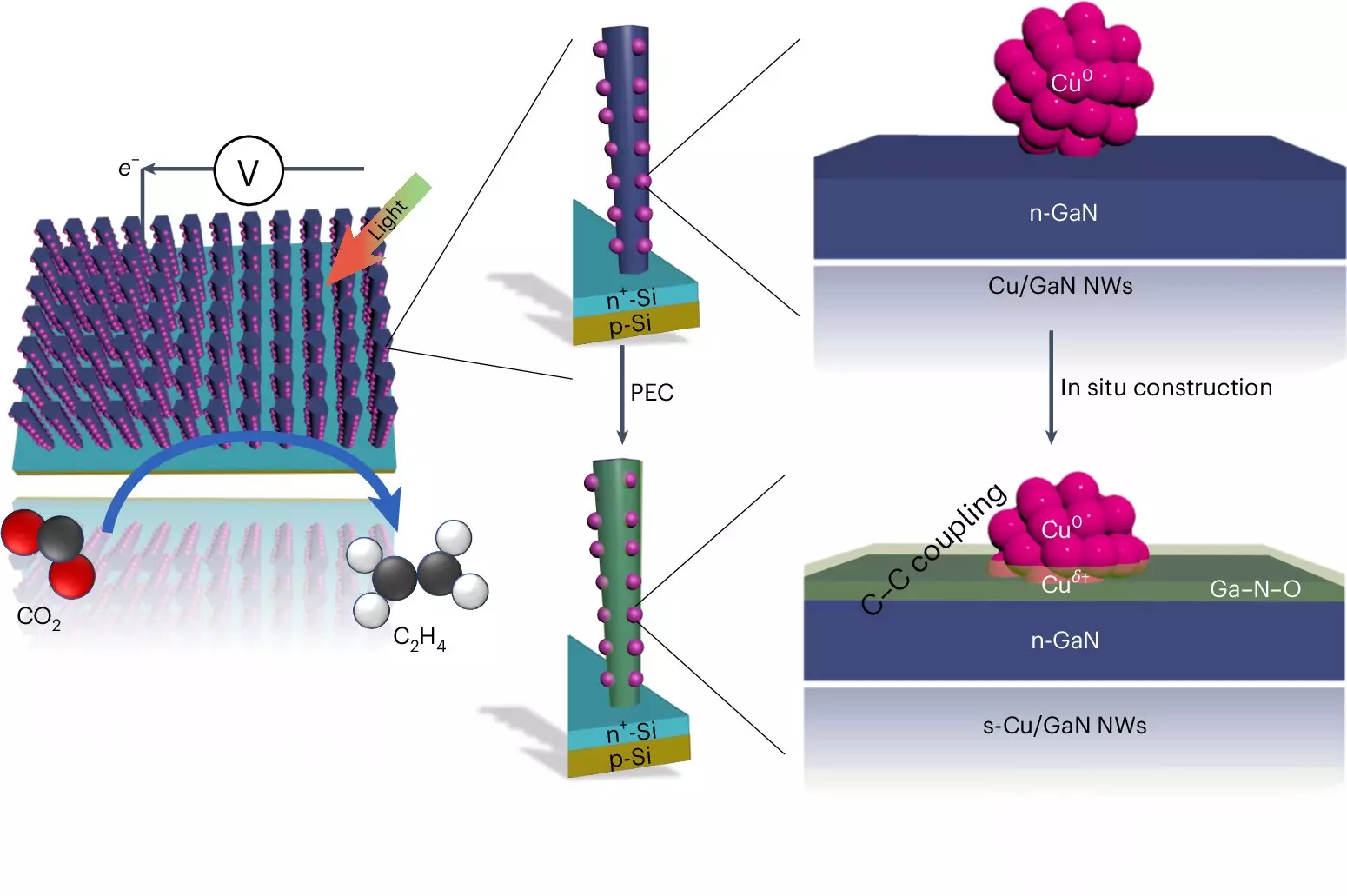
Central to the functionality of this artificial photosynthesis system is its adeptness in utilizing solar energy to initiate chemical reactions. The device incorporates advanced semiconductor technology, employing a structure of gallium nitride nanowires that are just 50 nanometers thick. These nanowires are positioned atop a silicon substrate and submerged in a carbon dioxide-enriched aqueous solution. The magic happens when sunlight strikes the surface, exciting electrons that facilitate the splitting of water molecules.
The process results in the generation of hydrogen, which is crucial for ethylene production, but also produces oxygen. Interestingly, the gallium nitride nanowires play a dual role in this system; they not only engage in water-splitting reactions but also capture freed oxygen atoms, transforming into gallium nitride oxide. This further enhances the catalytic efficiency of the system, underscoring the cleverness of its design—a harmony that supports its robust performance.
As the reaction proceeds, carbon dioxide molecules are transformed via copper clusters embedded within the nanowires. These clusters—each consisting of approximately 30 atoms—are adept at seizing hydrogen and sprawling carbon structures, engaging carbon monoxide in the process. With the energy from sunlight, two carbon monoxide molecules effectively bond, culminating in ethylene production.
One of the standout features of the University of Michigan’s artificial photosynthesis system is its extraordinary longevity and efficiency. While other catalytic systems have reported similar efficiencies (around 50%), they often require carbon-based fluids and can degrade significantly after just hours of operation. Remarkably, the Michigan system sustained continuous operation for 116 hours without efficiency loss, and similar configurations have achieved uptime of up to 3,000 hours.
This highlights not only the robustness of their design but also showcases an innovative synergy between the components. The interaction between gallium nitride and the ongoing water-splitting process lends the catalyst a self-healing property that enhances its durability, helping ensure steady production rates over extended periods.
Looking forward, the team’s aspirations expand beyond just ethylene. They aim to develop a range of multicarbon products, including heavier alcohols such as propanol, which could be pivotal in creating a sustainable, transportable liquid fuel supply. “Liquid fuels stand to revolutionize our existing transportation infrastructures, transitioning them into more sustainable alternatives,” notes Bingxing Zhang, a key contributor to the development.
The implications of this research are immense, pointing toward a future where CO2 emissions can be comfortably redirected away from harming the planet, instead contributing to productive uses. As technology continues to evolve, the University of Michigan’s work paves the way for a more sustainable approach to fuel production—one that may ultimately play a significant role in mitigating climate change while also addressing our perennial energy needs.

Leave a Reply