In the intricate world of molecular science, the phenomenon of electron transfer is fundamental to a multitude of processes, from the natural occurrence of photosynthesis in plants and bacteria to the engineered efficiency of solar panels through photovoltaics. At its core, electron transfer involves the movement of charge at the molecular level, a process intricate enough to be influenced by quantum mechanics and the nuanced dynamics of molecular behavior. Understanding the techniques and mechanisms underlying charge transfer not only enriches our comprehension of molecular interactions, but also serves as a pathway to enhancing technologies centered on energy conversion and storage.
The rapid shift of electronic density in molecules upon light absorption is a fascinating aspect of chemistry that occurs on incredibly short timescales, often measured in femtoseconds (10^-15 seconds) and even down to attoseconds (10^-18 seconds). The capability to capture these fleeting moments of electron and charge transfer is of paramount importance, as it reveals the hidden dynamics that govern the fundamental behavior of molecules. With advancements in ultrafast spectroscopy, scientists can now peer into the intricacies of these processes and glean insights that could revolutionize our approach to molecular engineering.
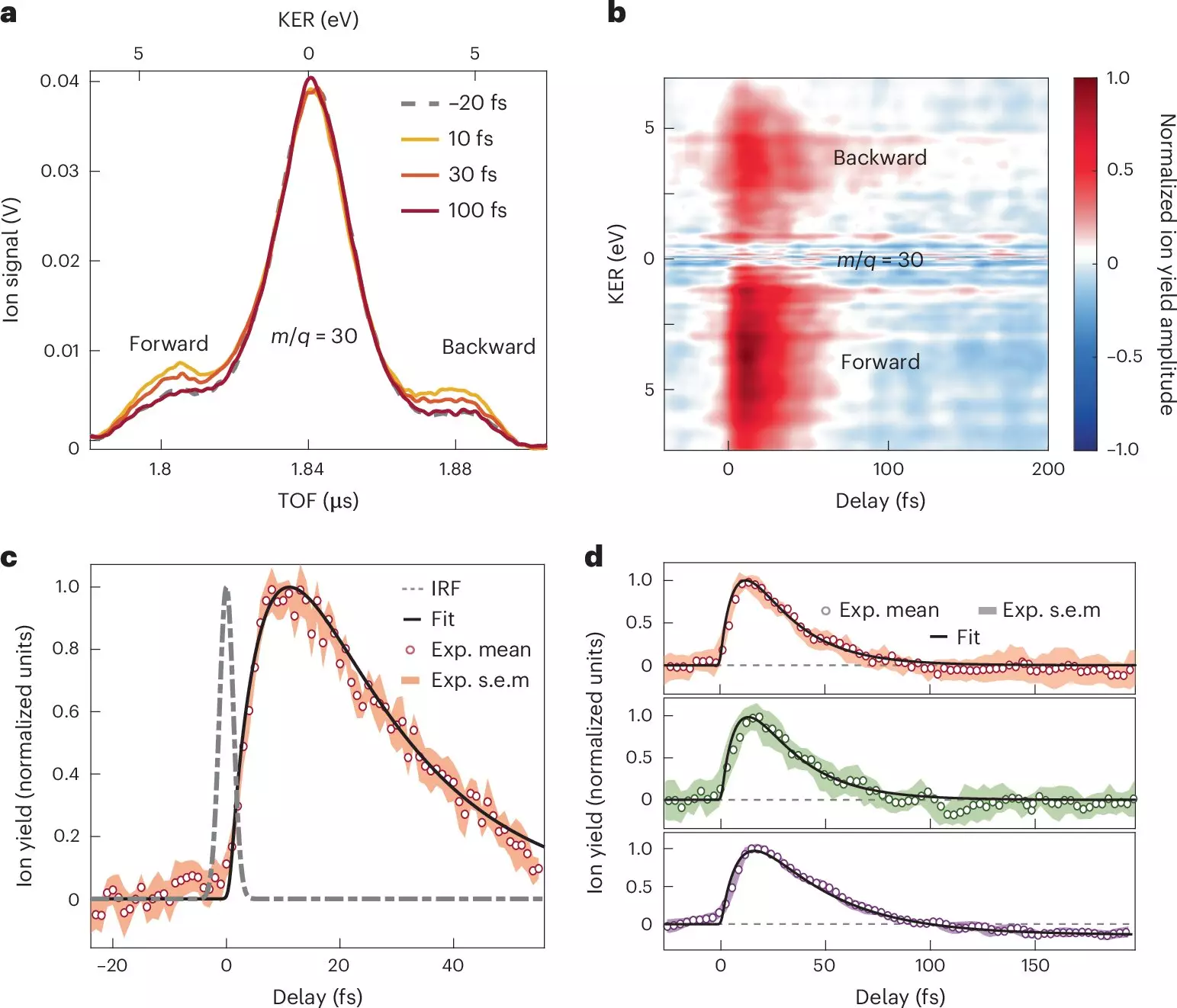
Recent research conducted by scientists at prominent institutions, including Politecnico di Milano and the Madrid Institute for Advanced Studies in Nanoscience, has harnessed the power of attosecond extreme-ultraviolet (XUV) pulses to explore the ultrafast dynamics of molecular systems. This pioneering study not only contributes significant knowledge to our understanding of electron dynamics but also uncovers the complexities of the interactions between electrons and atomic nuclei in charge transfer systems.
Utilizing a combination of advanced spectroscopic techniques, the researchers exposed nitroaniline molecules to highly sophisticated attosecond pulses. This allowed them to track the initial stages of electron transfer with an unprecedented temporal resolution. Their findings shed light on the rapid dynamics occurring in electron donor-acceptor systems, demonstrating that electron transfer can happen in less than 10 femtoseconds—a blink of an eye in the realm of atomic motions.
The study highlighted that the transfer of electrons from the amino group—acting as the donor—was followed by a relaxation phase that unfolded over a sub-30-femtosecond timescale. During this relaxation, the unfolding dynamics showcased how synchronized movements between electrons and atomic nuclei can significantly influence charge transfer processes. By gaining insight into these spontaneous transformations, scientists are beginning to grasp the structure-dynamics relationships that dictate how molecular architectures respond to photoionization.
Moreover, the research uncovered specific timescales related to the migration of charge from the electron donor to the adjacent chemical bond linked to a benzene ring. This knowledge could illuminate the traditional textbooks’ diagrams that qualitatively depict electron migration in organic compounds, providing a crucial bridge between theoretical models and practical observations.
The Implications of Ultrafast Studies on Future Research
The importance of this research transcends the immediate findings; it sets a robust foundation for future explorations in both theoretical modeling and practical applications of attosecond science. The insights garnered from the interaction of nitroaniline molecules not only advance our understanding of ultrafast processes but also open new avenues for engineering molecular systems with optimized electronic properties.
Further research may include investigating a broader range of molecules to determine if similar patterns of electron dynamics exist, potentially leading to generalized principles governing electron transfer in various chemical systems. As we continue to unlock the mysteries of molecular interactions, the integration of advanced spectroscopic tools will play a pivotal role in shaping our understanding and manipulation of molecular processes at an unprecedented speed.
The rapidly evolving field of ultrafast spectroscopy holds immense promise, as it enables us to not only visualize but also predict the dynamics of molecular charge transfer. By expanding our awareness of these fundamental chemical phenomena, we pave the way for innovative technologies that could capitalize on the efficiencies illustrated by nature—transforming how we harness energy and develop new materials for the future.

Leave a Reply